Transforming Our Breakthrough Science Through Research & Development

We are Accelerating Clinical Research to Address the Unmet Need in Wet AMD
At Opthea, we value collaboration with the retinal community to help shape the future of patient care. Our clinical programs are designed to translate our breakthrough science into products that will prevent or treat progressive retinal diseases that can cause blindness. In our completed clinical trials, we are already seeing promising results.
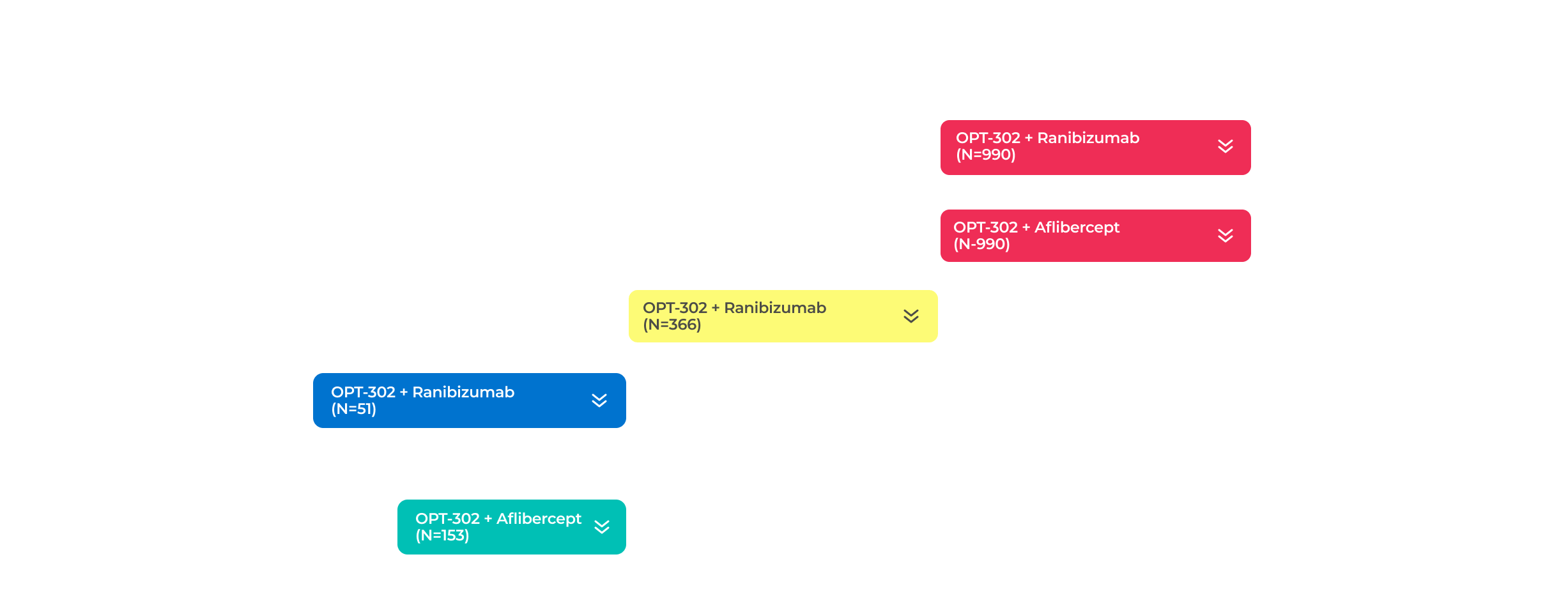
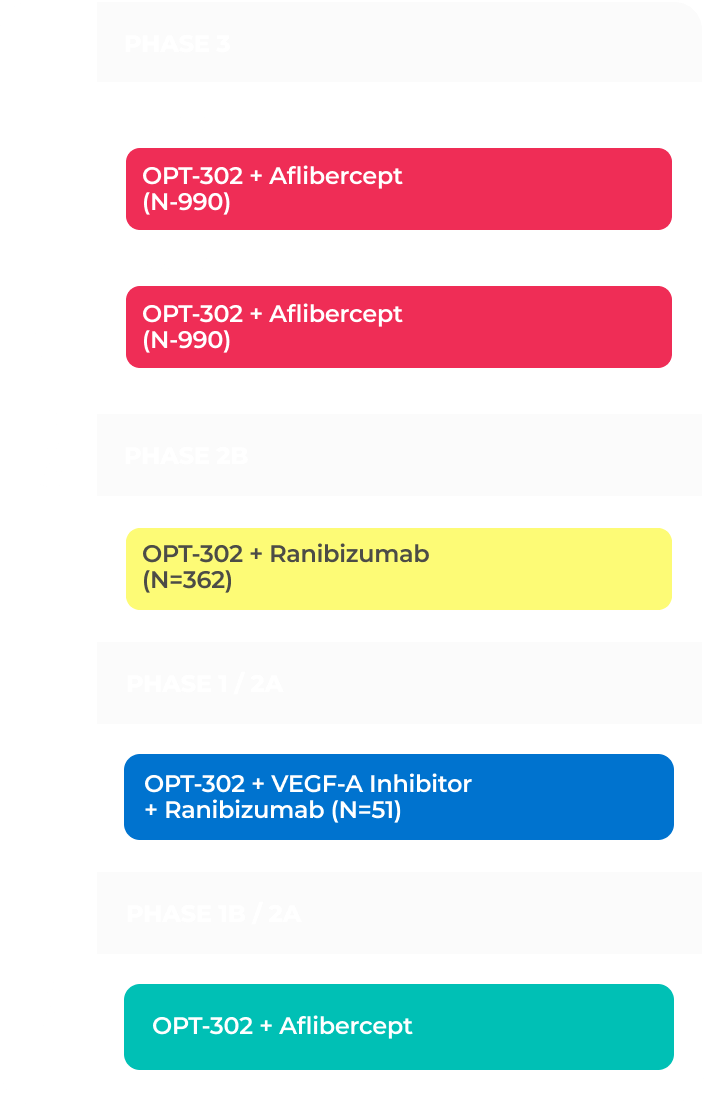
Wet Age Related Macular Degeneration Trials
OPT-302 is the first-in-class VEGF-C/D ‘trap’ in clinical development for wet AMD and it has also received fast track designation from U.S. Food and Drug Administration.22
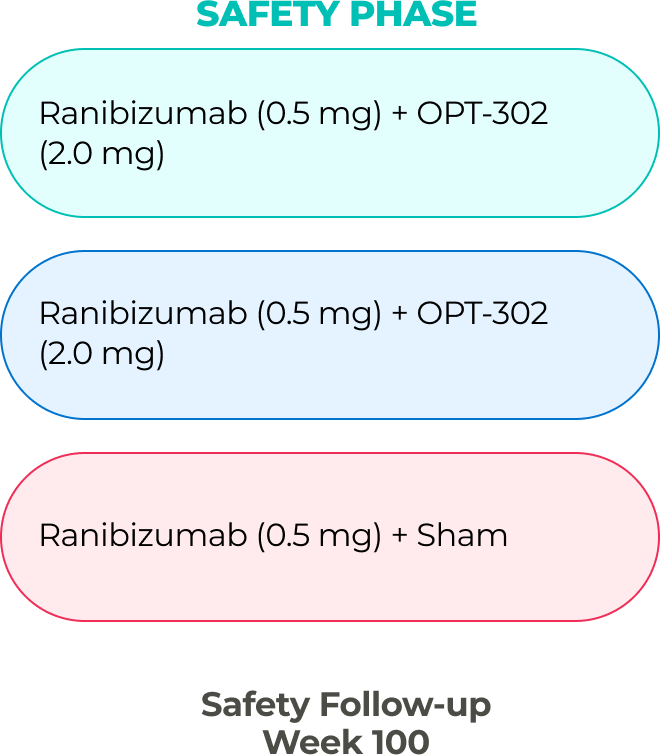
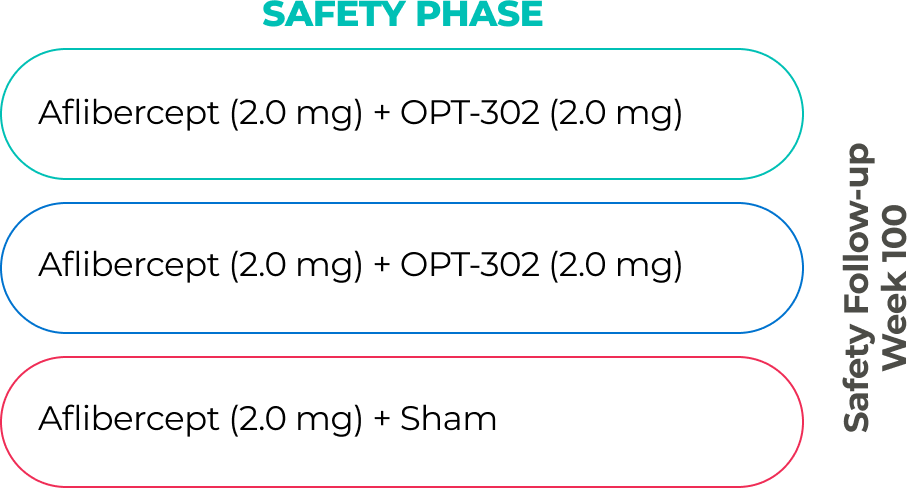
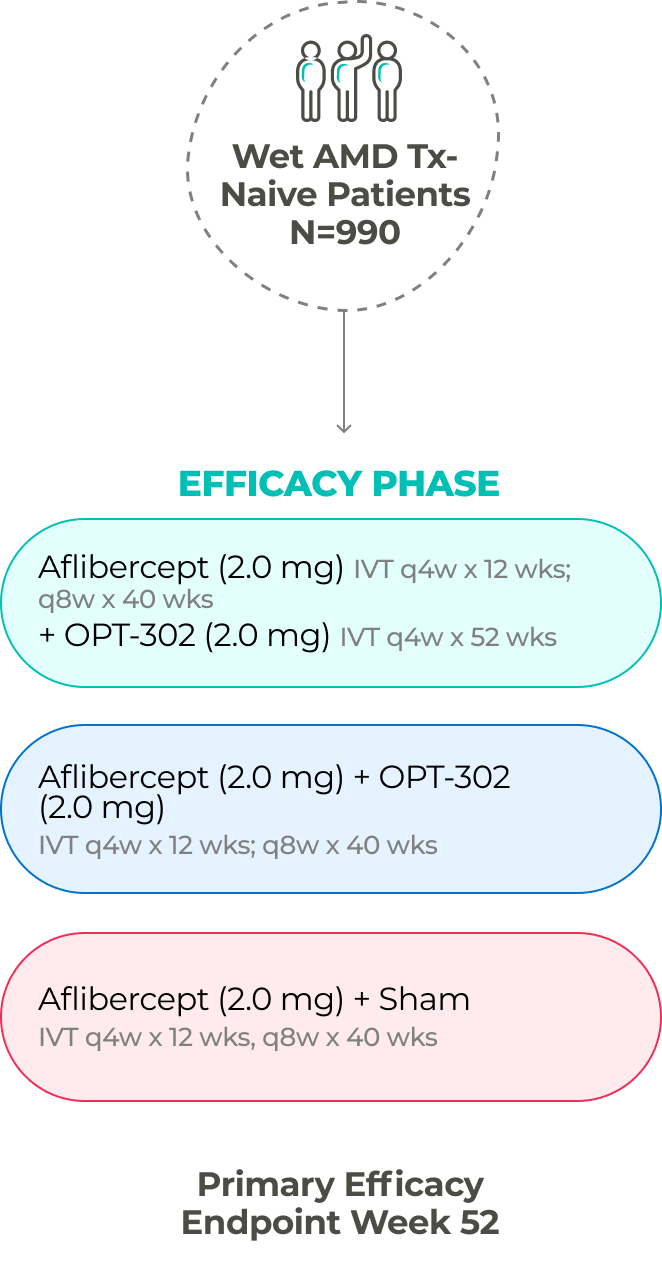
The company has two ongoing global Phase 3 registrational trials of OPT-302 in combination with standard-of-care anti-VEGF-A inhibitors.10,11,21
PHASE 3 (in-progress)
PHASE 2B (COMPLETE)
Results from a Phase 2B study of OPT-302 in combination with ranibizumab in treatment naïve patients with wet AMD
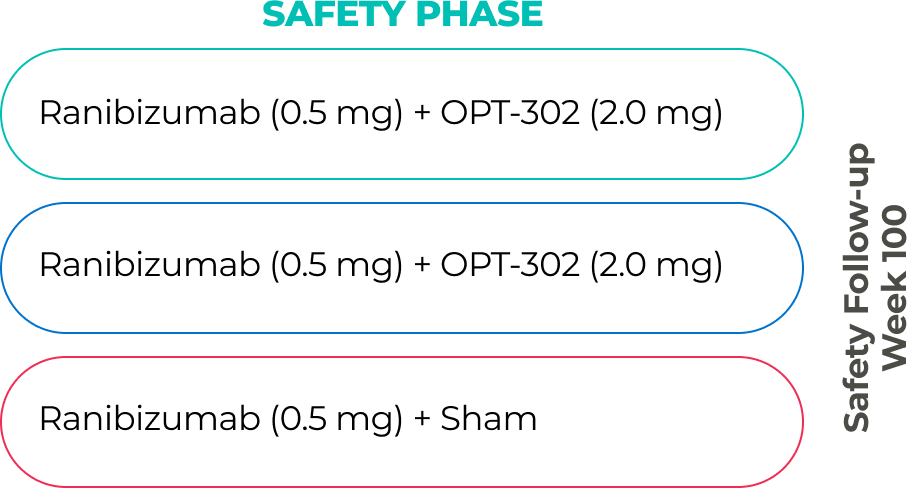
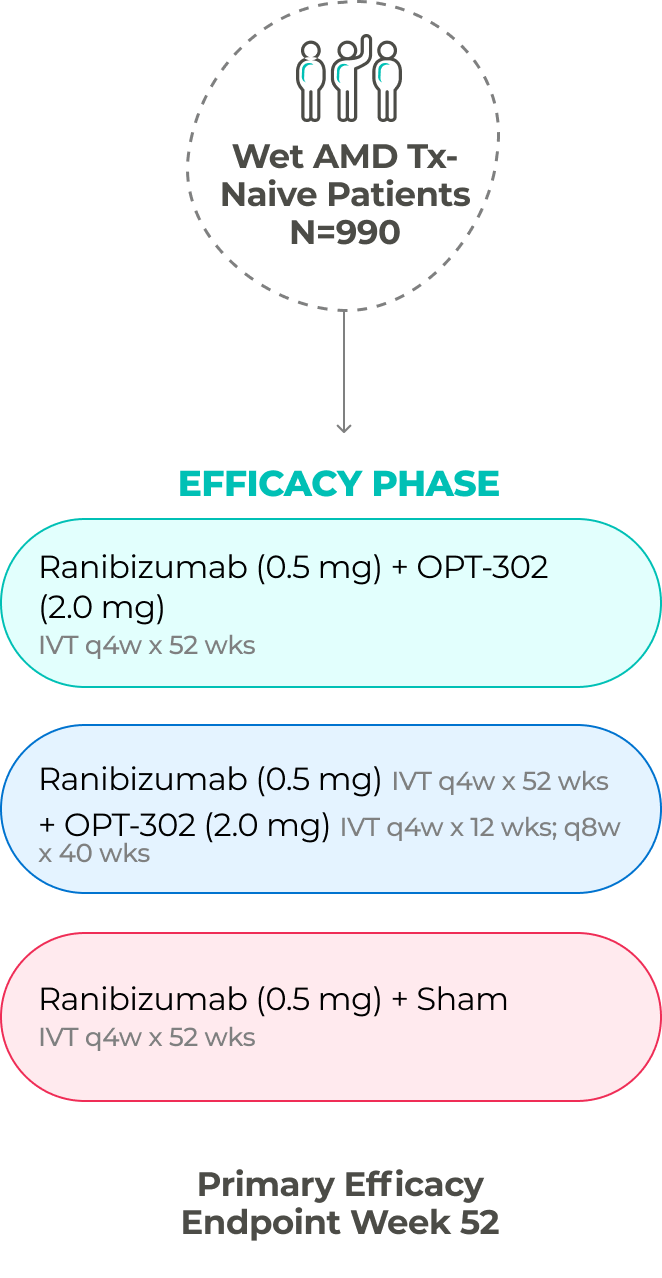
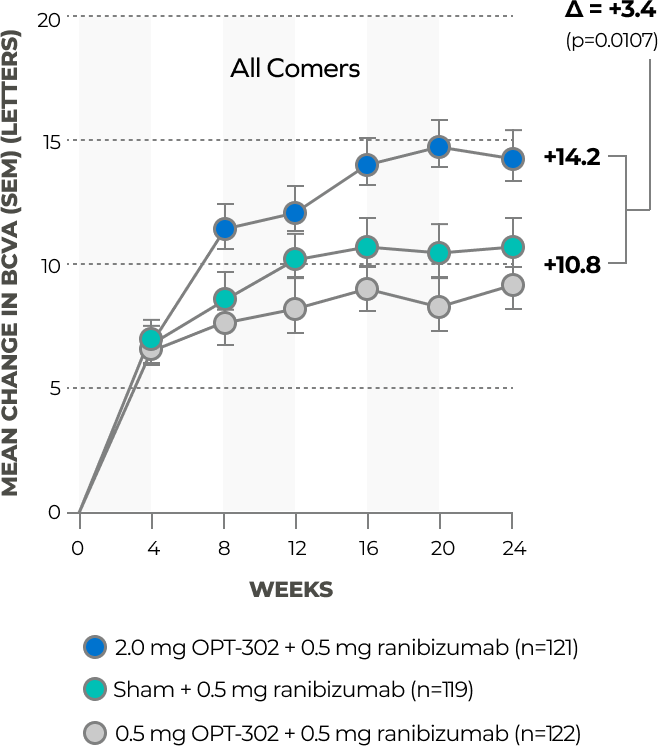
In a completed Phase 2B trial in 366 treatment naïve patients with wet AMD, intravitreal administration of OPT-302 (2.0 mg) in combination with ranibizumab (0.5 mg) met the primary endpoint of demonstrating statistically significant superiority in the mean gain in best corrected visual acuity (BCVA) from baseline to week 24 compared to ranibizumab (0.5 mg) monotherapy. These findings demonstrate the potential benefits of dual inhibition of VEGF-C/D and VEGF-A.9
OPT-302 (2 mg) + ranibizumab met the primary endpoint of superiority in mean gain in BCVA at 24 weeks versus ranibizumab monotherapy in the overall all-comer study population
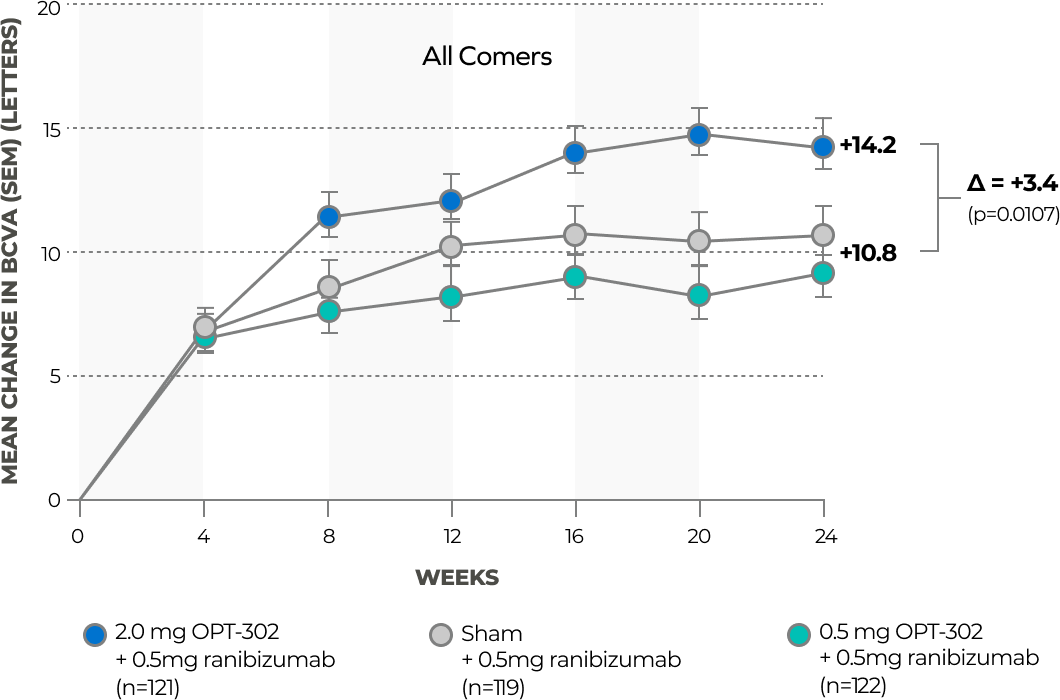
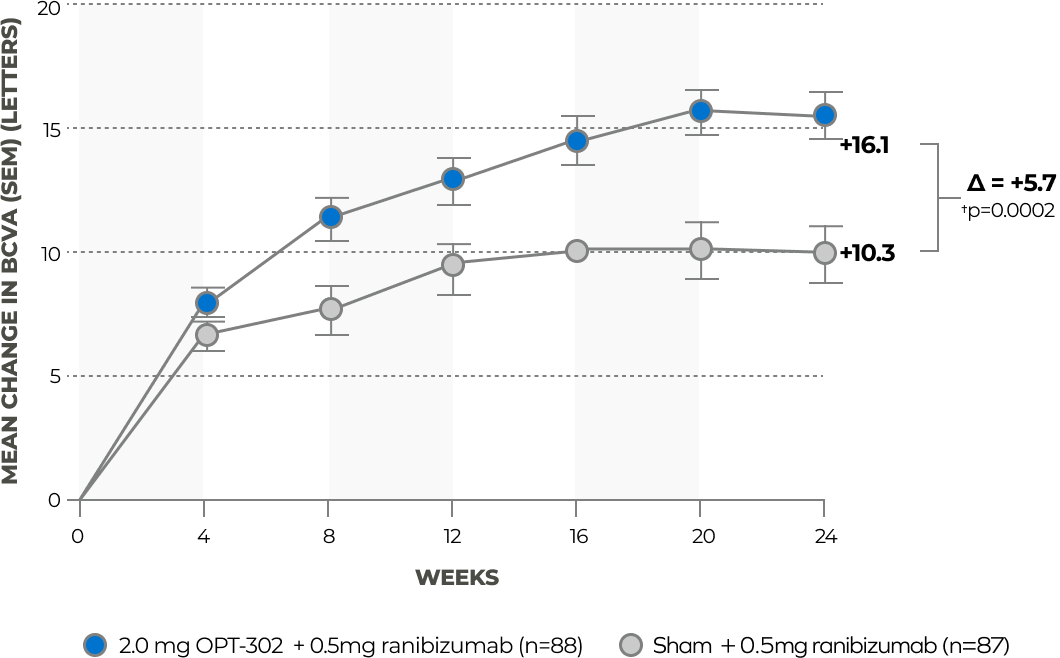
OPT-302 (2 mg) + ranibizumab demonstrated improved mean BCVA over ranibizumab monotherapy at 24 weeks in patients with minimally classic and occult lesions**†, 22
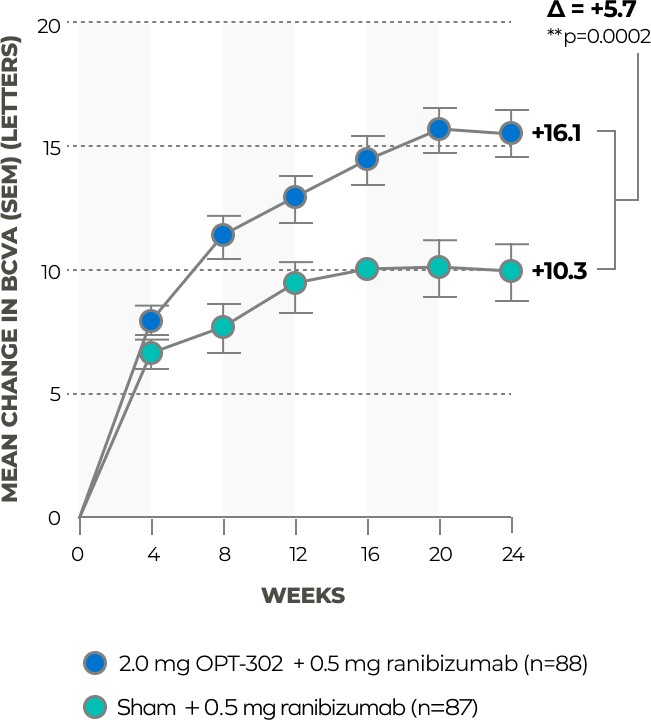
* The following sub-group analyses were pre-specified: Analysis of ETDRS BCVA at week 24 in patients classified by lesion type (predominantly classic, minimally classic and occult) and in subgroups with Retinal Angiomatous Proliferation (RAP) detected/not detected at baseline.
† RAP lesions were excluded from this analysis.
** Unadjusted p-value.
Safety Results from the Phase 2b Study
OPT-302 given in combination with ranibizumab was well tolerated and demonstrated a favorable safety profile comparable to ranibizumab standard of care monotherapy.9
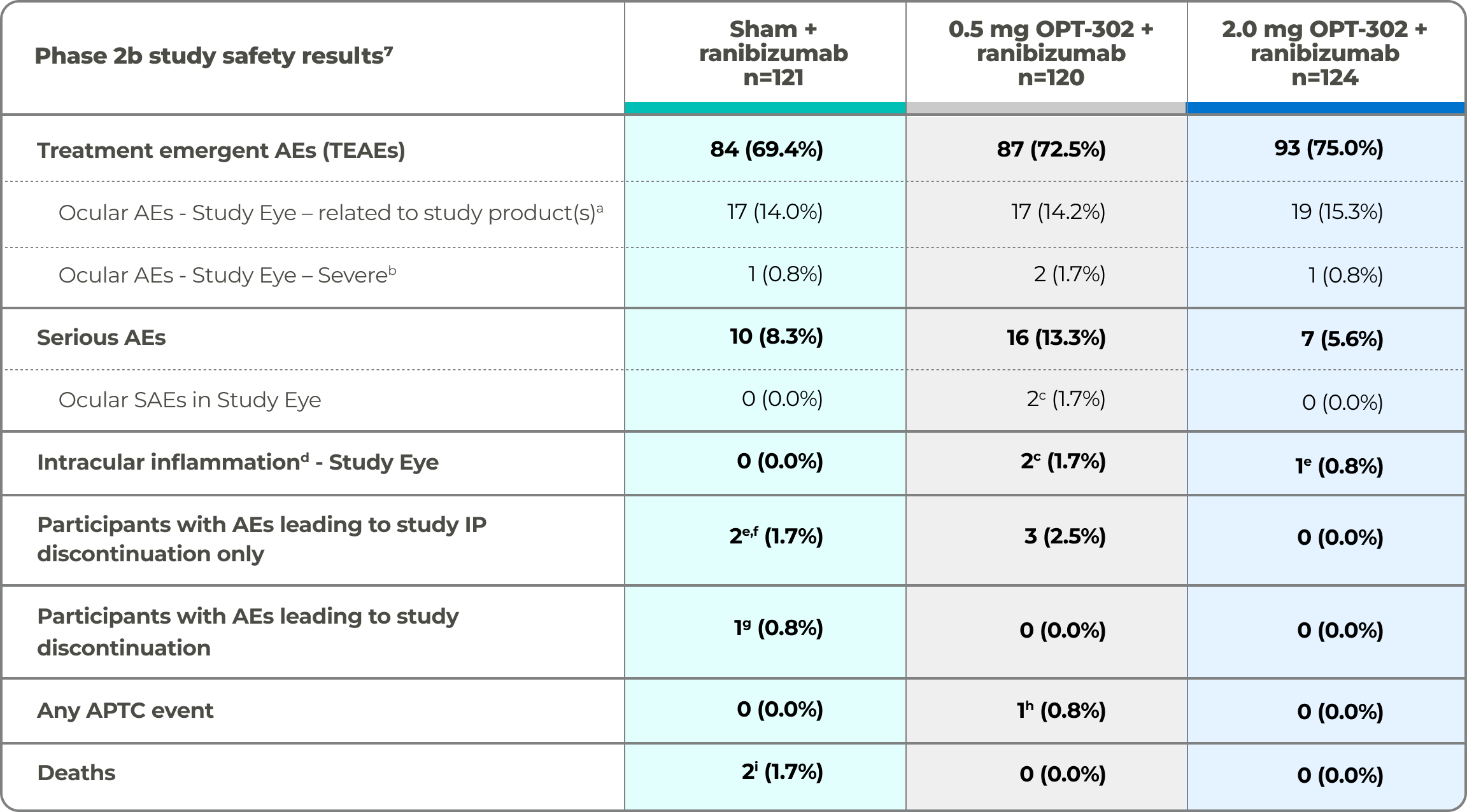
Phase 2b study safety results7
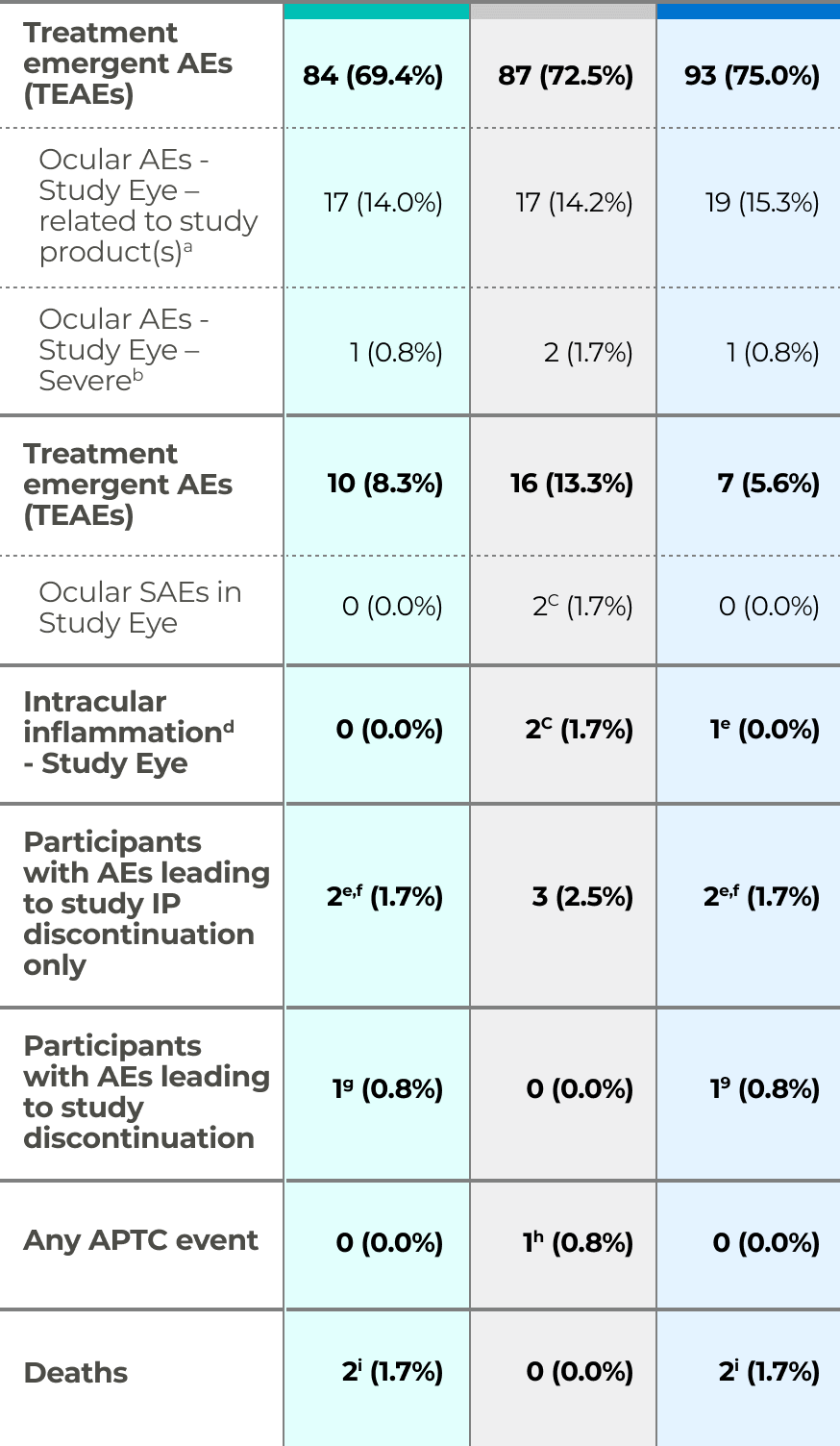
Safety population analyzed according to medication received. a. Assessed by investigator to be “possibly related”, “probably related” or “definitely related” to administration of study drug(s) b. Assessed by Investigator to be National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or above, or, if CTCAE grade is unavailable, an AE assessed as “causing an inability to perform normal daily activities” c. SAE of endophthalmitis, with AEs of hypopyon and anterior chamber cell (n=1), SAE of vitritis (n=1) d. AEs considered to be indicative of intraocular inflammation, defined prior to database lock as: Endophthalmitis, iritis, vitritis, iridocyclitis, uveitis, hypopyon, viral iritis, or anterior chamber inflammation e. Transient anterior chamber cell (trace 1-4 cells) f. Not reported as a TEAE g. Non-fatal myocardial infarction h. Pneumonia (n=1), infective endocarditis (n=1). h. Non-fatal myocardial infarction. i. Pneumonia (n=1), infective endocarditis (n=1).
References
Safety population analyzed according to medication received. a. Assessed by investigator to be “possibly related”, “probably related” or “definitely related” to administration of study drug(s) b. Assessed by Investigator to be National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or above, or, if CTCAE grade is unavailable, an AE assessed as “causing an inability to perform normal daily activities” c. SAE of endophthalmitis, with AEs of hypopyon and anterior chamber cell (n=1), SAE of vitritis (n=1) d. AEs considered to be indicative of intraocular inflammation, defined prior to database lock as: Endophthalmitis, iritis, vitritis, iridocyclitis, uveitis, hypopyon, viral iritis, or anterior chamber inflammation e. Transient anterior chamber cell (trace 1-4 cells) f. Not reported as a TEAE g. Non-fatal myocardial infarction h. Pneumonia (n=1), infective endocarditis (n=1). h. Non-fatal myocardial infarction. i. Pneumonia (n=1), infective endocarditis (n=1).
Results from a Phase 2B Study of OPT-302 in Patients with PCV
In the Phase 2B study, a total of 66 patients (18%) with Polypoidal Choroidal Vasculopathy (PCV) out of the total study population of 366 were included in a prespecified subgroup analysis. OPT-302 combination therapy demonstrated greater improvements in BCVA at 24 weeks ompared to ranibizumab monotherapy. The +6.7 letters comparative improvement in mean BCVA of 2 mg OPT-302 combination therapy over ranibizumab was accompanied by a greater improvement in secondary vision and anatomical outcome measures at week 24.12
PHASE 1/2A (COMPLETE)
Results from a Phase 1/2A Study of OPT-302 in Patients with Wet AMD
A Phase 1/2A study with 51 patients assessed the safety and tolerability of OPT-302 administered via ocular injection as a monotherapy and in combination with ranibizumab. The results showed that OPT-302 had a favorable safety profile as a monotherapy and in combination therapy with ranibizumab. Additionally, the secondary outcomes included assessments of clinical activity, and the combination therapy demonstrated gains of 10.8 letters in visual acuity.23
Gains in Best Correct Visual Acuity
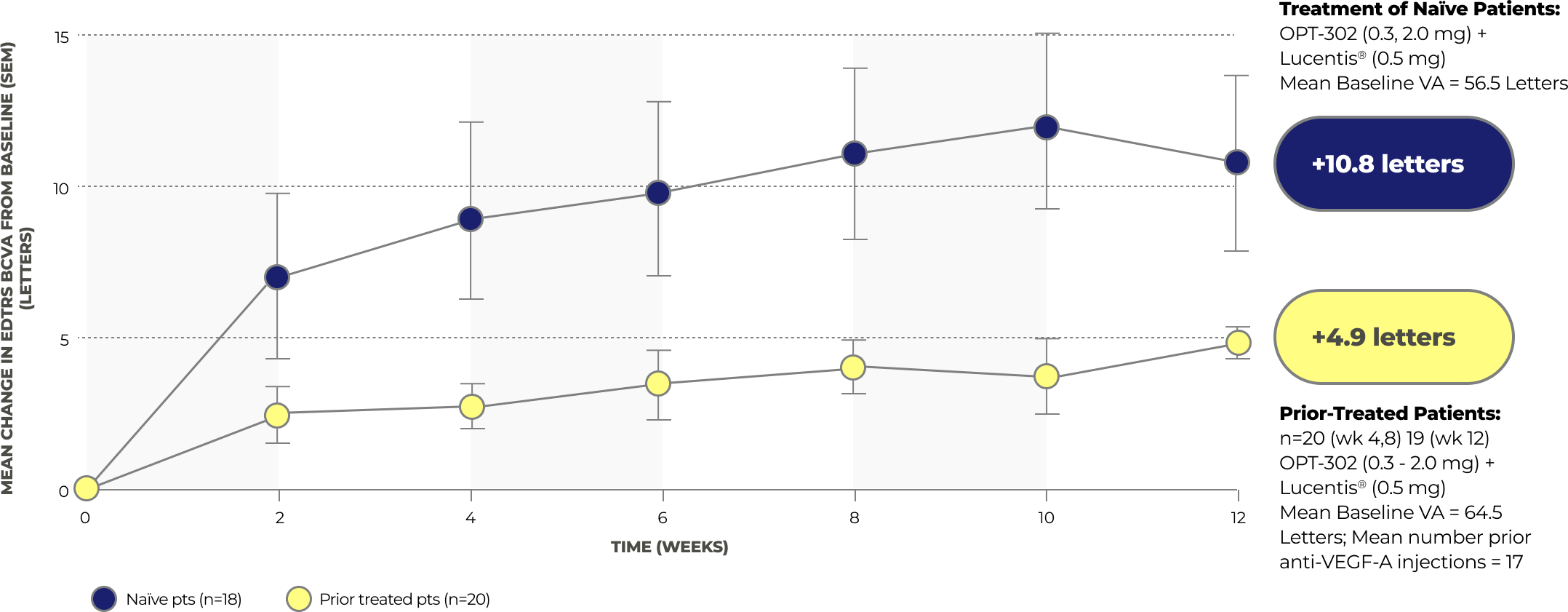
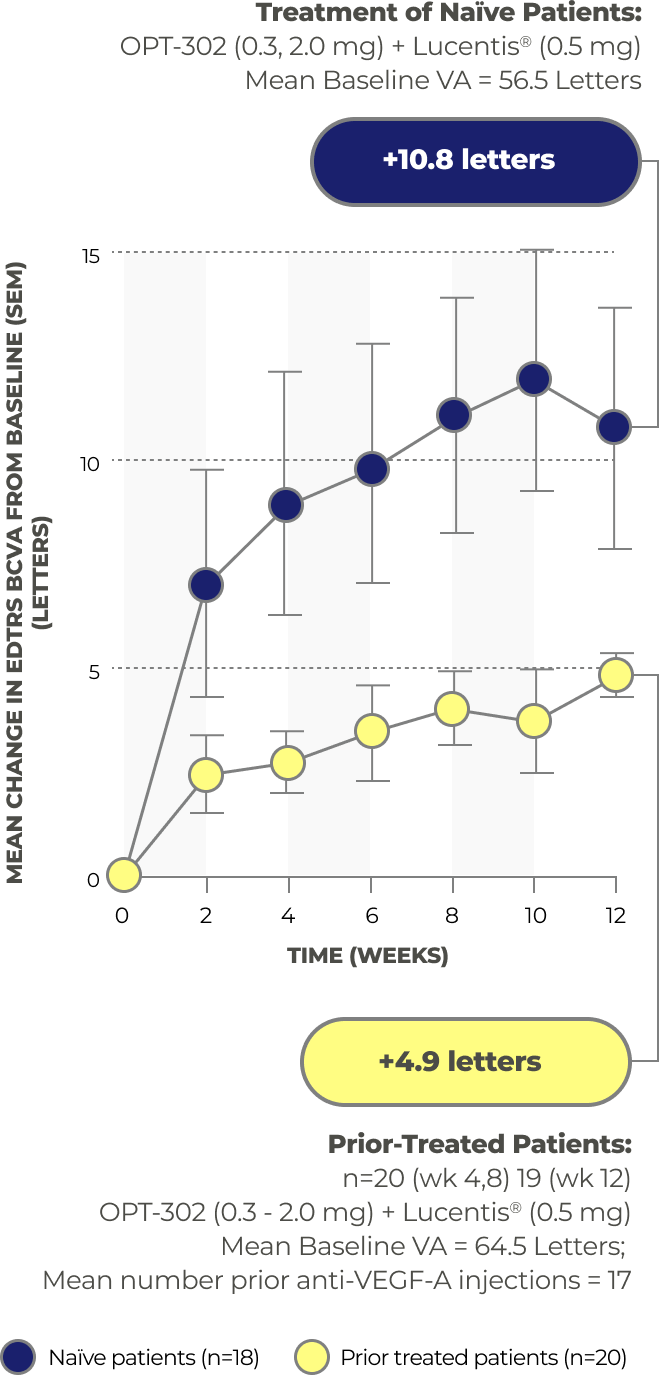
Safety Results from the Phase 1/2A Study
- Doses up to 2.0 mg of OPT-302 alone or in combination with ranibizumab generally was well tolerated.
- Favorable safety profile in both treatment naïve and patients previously treated with anti-VEFG-A drugs
- Most ocular adverse events were mild or moderate, manageable, temporary, and were related to the injection procedure rather than study drugs
- No dose-related adverse events were observed
- No significant adverse visual acuity safety signals were observed
- Low systemic exposure (No accumulation in serum after repeated injections)
- No dose-limiting toxicity
- No anti-drug antibodies or immunogenicity
Diabetic Macular Edema (DME)
PHASE 1B/2A (COMPLETE)
Results from a Phase 1B/2A Study of OPT-302 + aflibercept in Patients with persistent Central-involved DME
A Phase 1B/2A clinical trial administered OPT-302 in combination with aflibercept to 153 patients with central-involved diabetic macular edema (DME) who previously received standard of care anti-VEGF-A therapy. The trial had two parts. Phase 1B was a 9-patient dose-escalation study where OPT-302 (0.3, 1 or 2 mg) was administered in combination with aflibercept. The Phase 2A trial was a 144 patient dose expansion study that randomized patients 2:1 to receive either OPT-302 (2 mg) in combination with aflibercept (2 mg) of aflibercept (2 mg) monotherapy.23,24
Phase 1B: OPT-302 Dose Response in Visual Acuity Mean Gain at Week 12
A dose-response relationship of increased gains in best corrected visual acuity (BCVA) with ascending dose levels of OPT-302 combination treatment was observed.23
Phase 2A: Combination Therapy Resulted in More Mean Change in Vision Improvement at Week 12
The results demonstrated that eyes with persistent DME, which were sub-responsive to prior multiple prior doses of anti-VEGF-A therapy, demonstrated visual and anatomic improvement at 12 weeks following OPT-302 combination therapy.24
References
1. Lux A, Llacer H, Heussen F, et al. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol 2007;91:1318–1322. 2. Arsham Sheybani, Review of ophthalmology, 19 MARCH 2010. 3. Martin DF, Ophthalmology. 2012 Jul;119(7):1388-98. 4. Ehlken, C., Eye 28, 538–545 (2014). 5. Rosenfeld PJ, N Engl J Med. 2006 Oct 5;355(14):1419-31. 6. Heier JS, Ophthalmology. 2012;119:2537–48. 7. Steinkuller, P. American Medical Association Journal of Ethics. 2010; (12): 938-940. 8. Dugel PU, Ophthalmol Retina. 2020 Mar;4(3):250-263. 9. Timothy L. Jackson, PhD DOI: https://doi.org/10.1016/j.ophtha.2023.02.001. 10. Clinicaltrials.gov. OPT-302 (ShORe). https://clinicaltrials.gov/ct2/show/NCT04757610. 11. Clinicaltrials.gov. OPT-302 (COAST). https://clinicaltrials.gov/ct2/show/NCT04757636. 12. Arepalli S and Kaiser P. Int J Retin Vitr. 55(2021). 13. Cabral T,. Ophthalmol Retina. 2018 Jan;2(1):31-37. 14. Lieu C, PloS One. 2013; 8: e77117. 15. Li D, Cancer Lett. 2014; 346:45-52. 16. Rose S, Clin Neurosurg. 2010; 57: 123-8. 17. Fan F, Br J Cancer. 2011. 18. Puddu A, Exp Eye Res. 2016 May;146:128-36. 19. Bradford A. Moffat, Clin Cancer Res 2006;12(5) 1525-1532. 20. Claus Cursiefen, The Journal of Clinical Investigation Volume 113 Number 7 April 2004. 21. Zhou et al. BMC Ophthalmology (2020) 20:15. 22. https://www.globenewswire.com/en/news-release/2021/07/06/2258087/0/en/Opthea-s-OPT-302-Granted-FDA-Fast-Track-Designation-for-Wet-Age-Related-Macular-Degeneration.html 23. Boyer DS, American Academy of Ophthalmology annual meeting, Retina Subspecialty Day Vision for the Future; Nov. 13, 2020. 24. Boyer DS, meeting, Diabetic Retinopathy Symposium; July 24-26, 2020.