Opthea is a global biopharmaceutical company dedicated to improving and protecting vision in people with retinal disease
Opthea is boldly investigating the unmet medical need among patients with wet AMD who are receiving anti-Vascular Endothelial Growth Factor (VEGF)-A monotherapy:
%
of patients eyes fail to response or only partially respond1,2
%
of patients eyes fail to response or only partially respond1,2
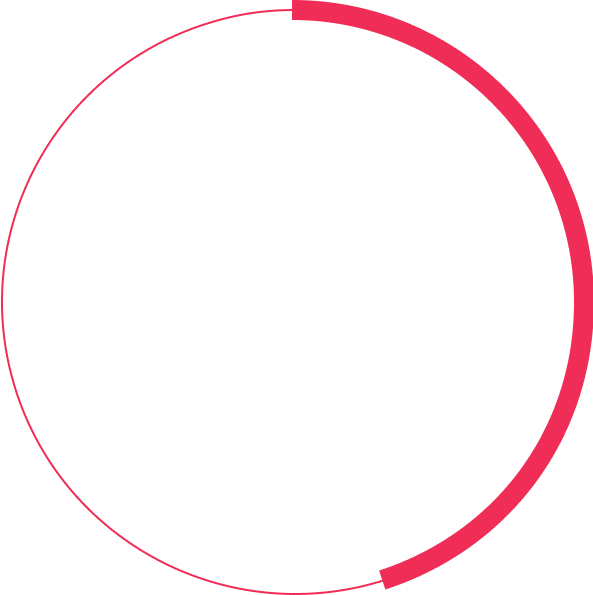
>%
of patients have persistent fluid after two years of treatment3
>%
of patients have persistent fluid after two years of treatment3
Up to
%
of patients suffer further vision loss despite anti-VEGF-A treatment4
Up to
%
of patients suffer further vision loss despite anti-VEGF-A treatment4
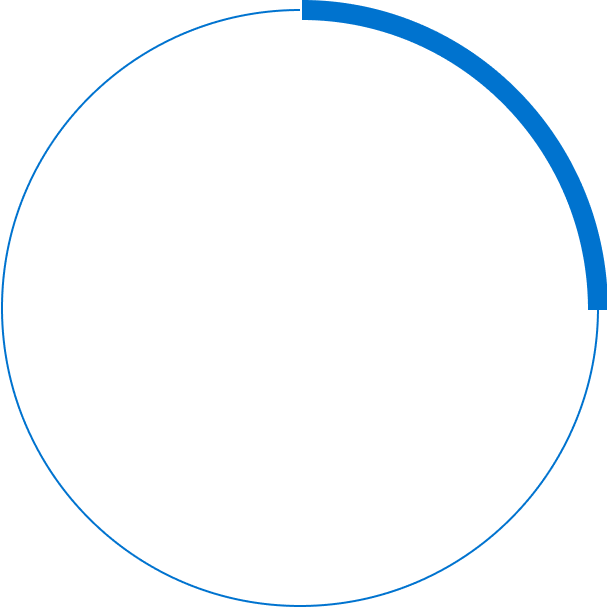
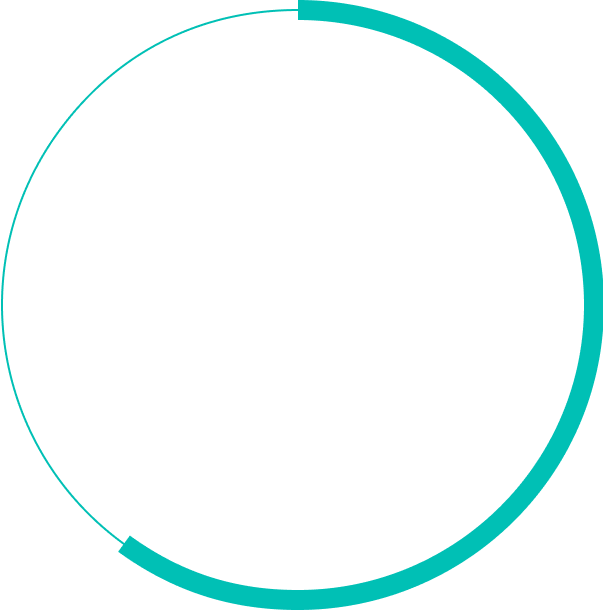
%
of patients fail to achieve 20/40 and most patients cannot resume daily activities such as driving or reading5,6,7
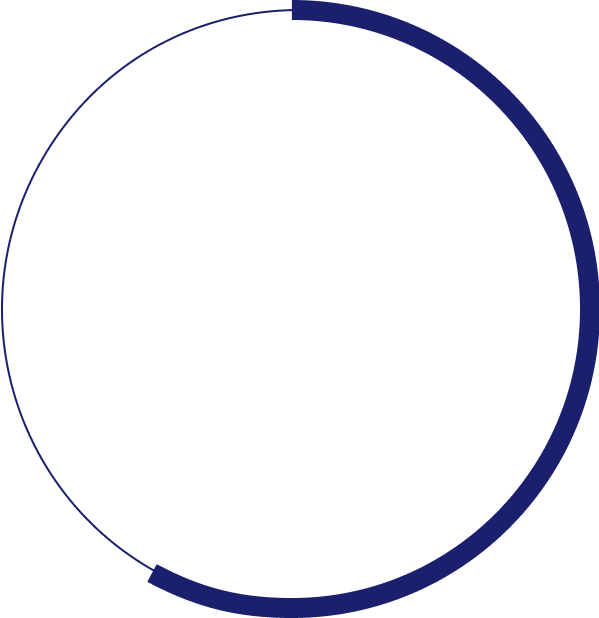
%
of patients fail to achieve 20/40 and most patients cannot resume daily activities such as driving or reading5,6,7
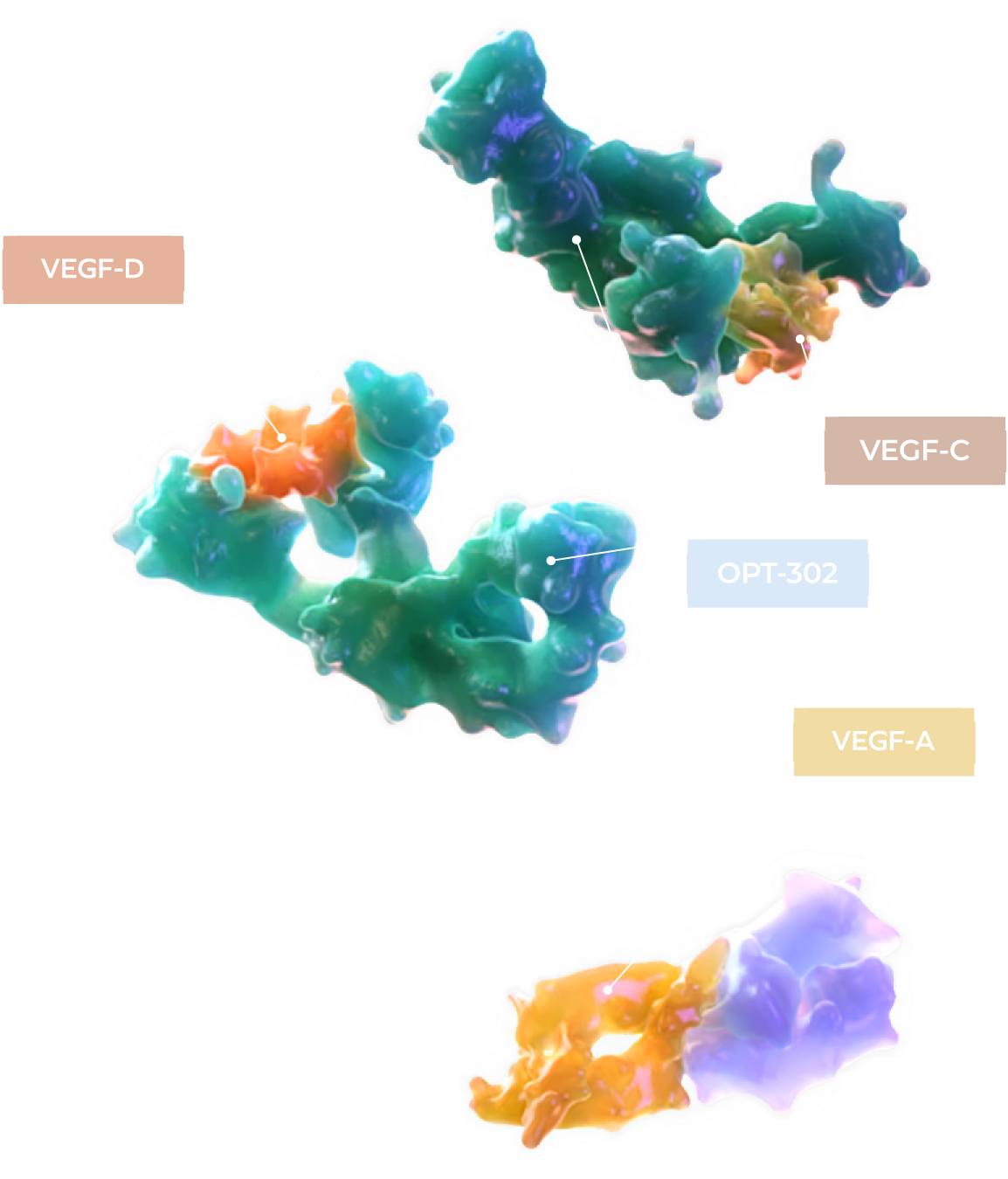
Differentiated Science Based on Bold Innovation
Our lead investigational drug candidate OPT-302, is a first-in-class molecule with a novel anti-VEGF-C / VEGF-D mechanism of action for use in combination with existing standard-of-care anti-VEGF-A inhibitors.8
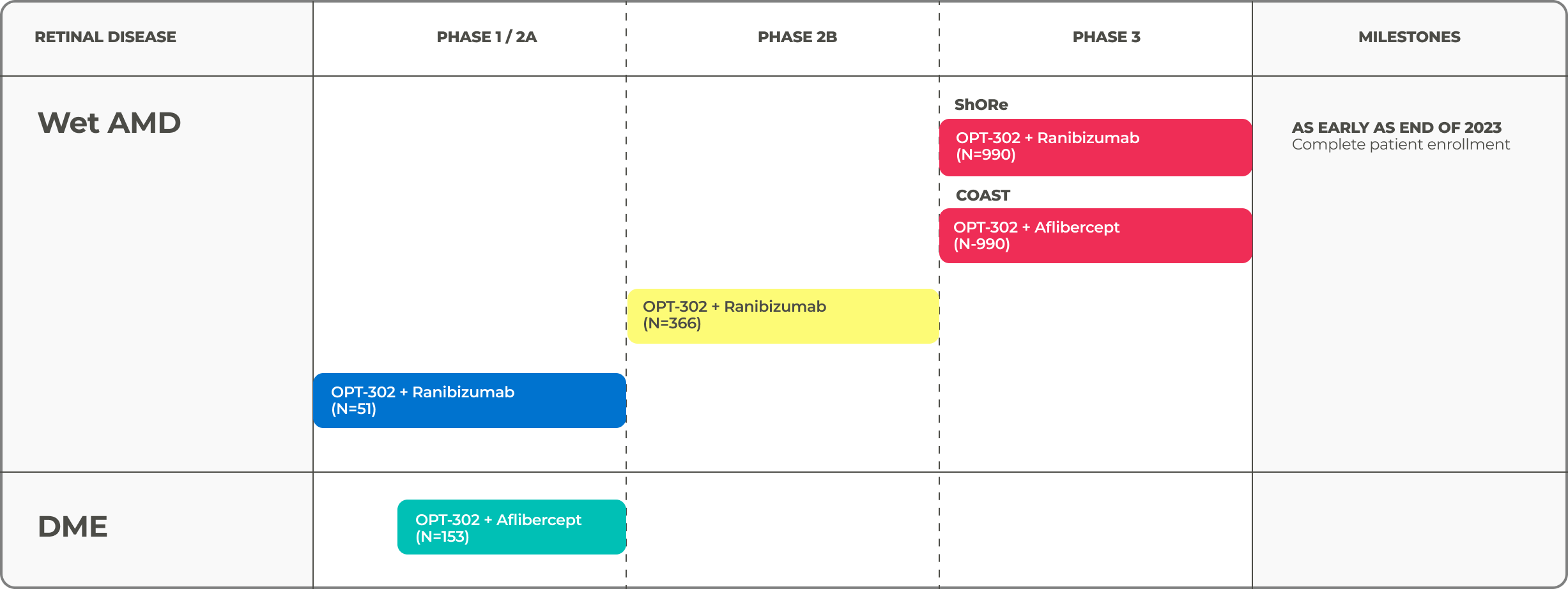
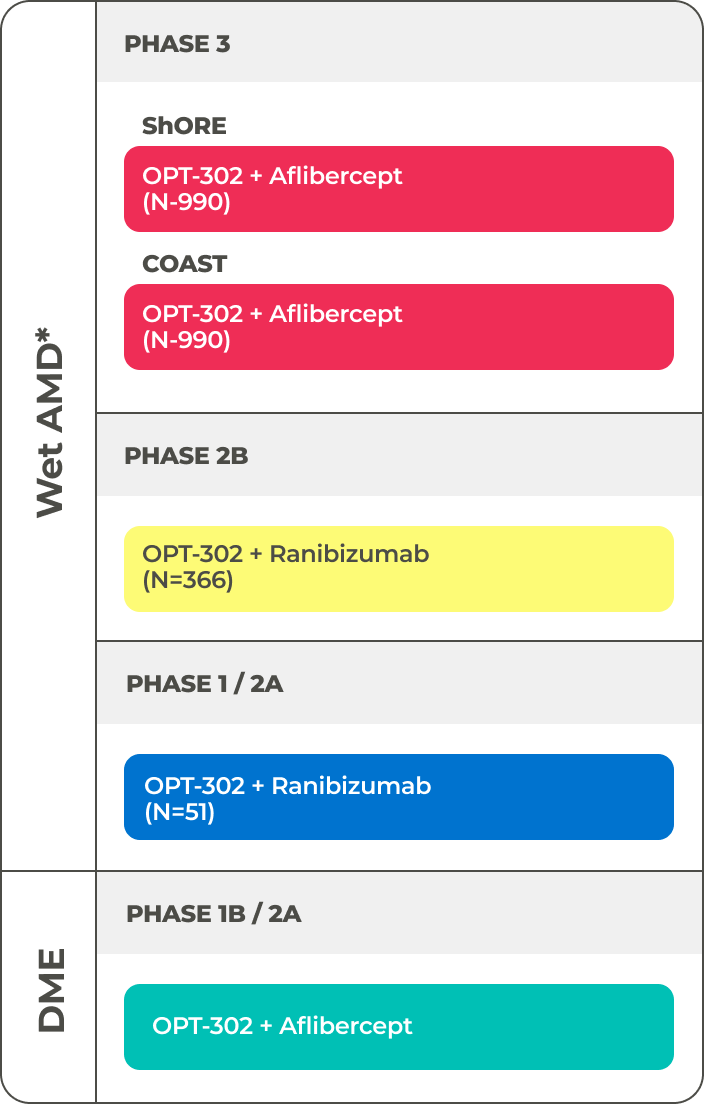
Clinical Trials
OPT-302 has the potential to become the first and only anti-VEGF-C / VEGF-D treatment for wet AMD and other retinal diseases. After meeting the primary endpoint in the Phase 2B wet AMD trial,9 the company is accelerating its late-stage clinical development of OPT-302 and has two ongoing Phase 3 clinical trials known as ShORe (OPT-302 + ranibizumab) and COAST (OPT-302 + aflibercept) that are each enrolling 990 treatment naïve patients with wet AMD.10,11
*WET AmD MILESTONES
- As early as end of 2023 Complete patient enrollment
Press Releases
Corporate Presentations
Equip yourself with the necessary tools to deliver a compelling presentation on Opthea’s latest news and updates that will effectively engage and inform your audience.
Investors
Our science-focused collaborative culture is unlocking long-term value for patients, healthcare providers, employees, and shareholders.
References
1. Lux A, Llacer H, Heussen F, et al. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol 2007;91:1318–1322. 2. Arsham Sheybani, Review of ophthalmology, 19 MARCH 2010. 3. Martin DF, Ophthalmology. 2012 Jul;119(7):1388-98. 4. Ehlken, C., Eye 28, 538–545 (2014). 5. Rosenfeld PJ, N Engl J Med. 2006 Oct 5;355(14):1419-31. 6. Heier JS, Ophthalmology. 2012;119:2537–48. 7. Steinkuller, P. American Medical Association Journal of Ethics. 2010; (12): 938-940. 8. Dugel PU, Ophthalmol Retina. 2020 Mar;4(3):250-263. 9. Timothy L. Jackson, PhD DOI: https://doi.org/10.1016/j.ophtha.2023.02.001. 10. Clinicaltrials.gov. OPT-302 (ShORe). https://clinicaltrials.gov/ct2/show/NCT04757610. 11. Clinicaltrials.gov. OPT-302 (COAST). https://clinicaltrials.gov/ct2/show/NCT04757636. 12. Arepalli S and Kaiser P. Int J Retin Vitr. 55(2021). 13. Cabral T,. Ophthalmol Retina. 2018 Jan;2(1):31-37. 14. Lieu C, PloS One. 2013; 8: e77117. 15. Li D, Cancer Lett. 2014; 346:45-52. 16. Rose S, Clin Neurosurg. 2010; 57: 123-8. 17. Fan F, Br J Cancer. 2011. 18. Puddu A, Exp Eye Res. 2016 May;146:128-36. 19. Bradford A. Moffat, Clin Cancer Res 2006;12(5) 1525-1532. 20. Claus Cursiefen, The Journal of Clinical Investigation Volume 113 Number 7 April 2004. 21. Zhou et al. BMC Ophthalmology (2020) 20:15. 22. https://www.globenewswire.com/en/news-release/2021/07/06/2258087/0/en/Opthea-s-OPT-302-Granted-FDA-Fast-Track-Designation-for-Wet-Age-Related-Macular-Degeneration.html 23. Boyer DS, American Academy of Ophthalmology annual meeting, Retina Subspecialty Day Vision for the Future; Nov. 13, 2020. 24. Boyer DS, meeting, Diabetic Retinopathy Symposium; July 24-26, 2020.